How to Determine Solubility in Water of Organic Compounds
One physical property that has links to intermolecular forces is solubility. Acids and amines are more soluble than nonpolar compounds due to H-bonding.

Solubility Of Organic Compounds Video Khan Academy
To verify that a compound has dissolved add 5 HCl to the NaOH mixture until the solution is acidic to pH paper.

. But depending upon the type of bond involved organic compounds may be soluble or not. Look for a precipitate indicating that the water-soluble salt has converted back into the water-insoluble compound. That is polar compounds usually are soluble in water and non-polar compounds are not soluble in water.
From this exercise you should learn which functional groups enhance water-solubility and how the size and shape of the organic compound influences water-solubility. If hydrophillic region is stronger than hydrophobic region molecule is soluble. Structure determines the lipophilicity.
However because of various properties of carbon-containing compounds in liquid samples the manner of preliminary sample treatment as well as the instrument settings will determine which forms of carbon are actually measured. As a rough estimate remember that water soluble organic compounds must have an oxygen or nitrogen containing functional groups. This also applies to organic compounds that can exist as ions.
Water-soluble organic compounds include organic anions dicarboxylic acids oxo acids dicarbonyls carbohydrates amino acids aliphatic amines urea and some miscellaneous multifunctional compounds containing multiple hydroxy carboxyl and carbonyl groups eg. Solubility of amines decreases as the basicity decreases. About Compounds Organic How Of To Solubility Determine.
For example sodium acetate consists of Na and CH3COO-ions which are highly soluble in water. Many ionic compounds are highly soluble in water because of the strong attraction between ions and the highly polar water molecules. Carboxylic acids are soluble in water because they can form hydrogen bonds with water.
O2 LiCl Br2 CH3OH Like dissolves like. An ionic compound that does dissolve in water is said to be soluble2 The result is an aqueous solution. To predicte solubility for a compound you can start from the fact that says Like dissolve like therefor for polar compound you are looking for polar.
The next very polar solvent which is also an organic compound is methanol. Actually only those compounds either inorganic or organic are soluble which interact with water. In fact what happens is that they just mix together and you cant even tell the difference except you can tell the difference the next morning.
Water-soluble compounds are tested with pH paper to see if they are acidic or basic. An understanding of the various types of noncovalent intermolecular forces allows us to explain many observable physical properties of organic compounds on a molecular level. Solubility rules are qualitative rules to determine whether an ionic compound will or will not dissolve in water at 25C.
Apr 02 2019 Arrange the following compounds in order of increasing solubility in water. Salts are extremely polar and are usually water soluble. How do you determine the solubility of organic compounds.
Whether some organic substance will dissolve in a liquid solvent and to what extent it will do so is linked to the. Water Soluble Compounds pH 8 pH Sodium Benzoate H2O. O2 LiCl Br2 CH3OH Like dissolves like.
Apr 02 2019 Arrange the following compounds in order of increasing solubility in water. That is polar compounds usually are soluble in water and non-polar compounds are not soluble in water. You will be determining the amount of water needed to dissolve 10 mL of different organic compounds.
You know that obviously you werent just drinking water. This also applies to organic compounds that can exist as ions. Another context low solubility organic compound in water indirecty represent volatile oil in that sense weakly volatile oilthermally labile compounds not anneable to.
Glyceraldehyde malic acid citric acid lactic acid. Many tertiary amines are more soluble in cold than in hot water at lower temperatures the solubility of the hydrates is involved. But when alkyl group gets larger solubility decreases.
In any case if the molecule has more than 3-4 carbon atoms its solubility is going to significantly decrease. Remember that carboxylic acids dissolve in water and. In general organic compounds are not soluble in water.
Which organic compounds is soluble in water. That has to do with the fact that both have the same polarity or similar polarity. Lets just expand this a little bit.
The highly polar water molecules. Although there are some exceptions you m ay assume in this experiment that all organic compounds that are in the ionic form will be water-soluble. Ad Over 27000 video lessons and other resources youre guaranteed to find what you need.
Think about it water. Formic acid ethanoic acid propanoic acids are water soluble. For example sodium acetate consists of Na and CH3COO- ions which are highly soluble in.

Chemistry The Central Science Chapter 13 Section 3
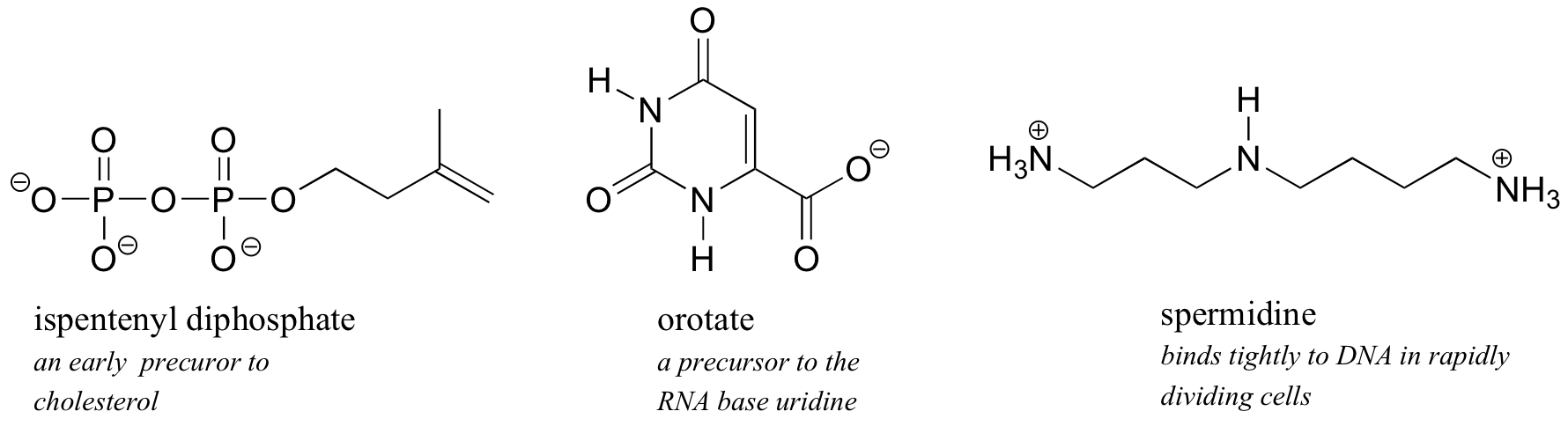
1 6 Physical Properties Of Organic Compounds Chemistry Libretexts
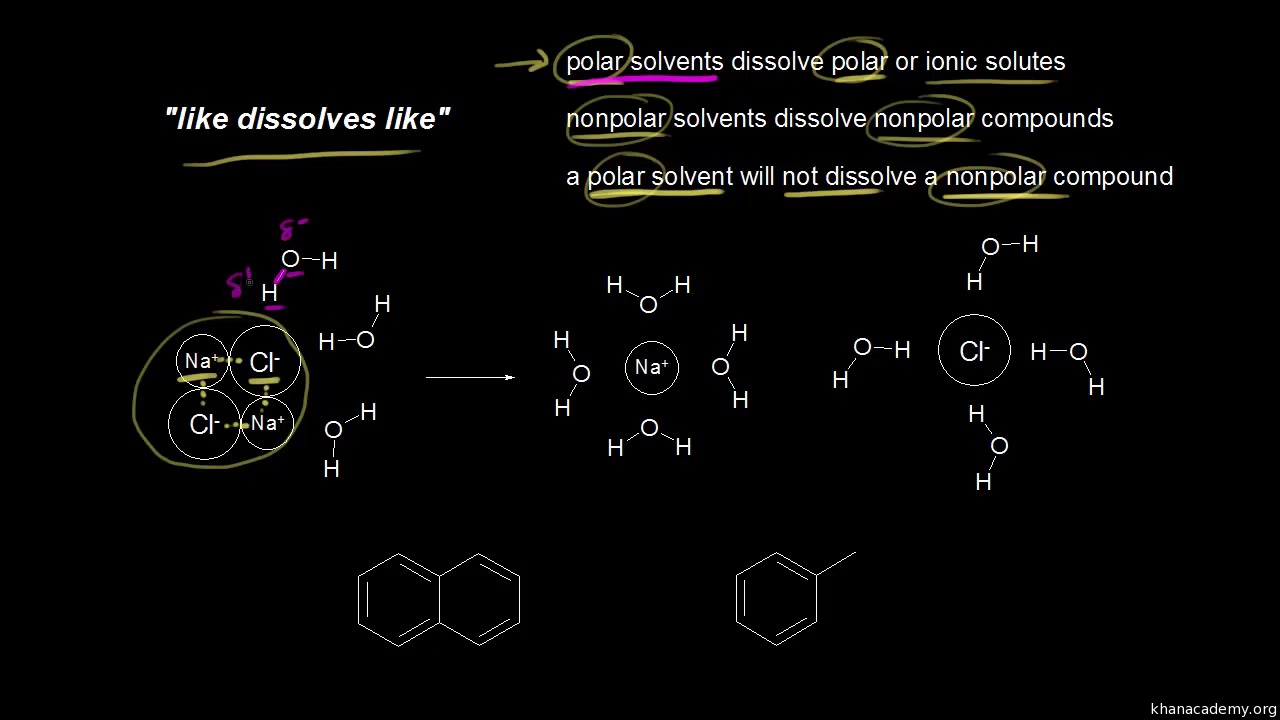
Comments
Post a Comment